Rutgers Saliva Test Approved One Year Ago
RUCDR Infinity Biologics pioneered the world’s first coronavirus saliva test
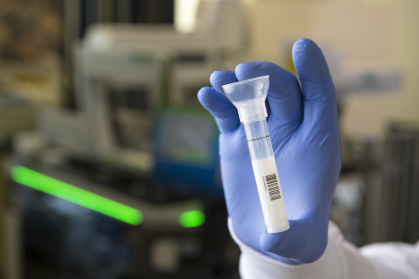
In the last year, a small vial that people fill to a line with saliva has become a widespread weapon in the fight to slow the spread of the COVID-19 pandemic.
Last April, when the SARS-CoV-2 coronavirus that causes COVID-19 was spreading rapidly, partly because testing resources were scarce, Rutgers scientists introduced the game-changing innovation. Since then, more than six million Rutgers saliva tests have been performed.
“The pioneering test has saved health care workers from unnecessary exposure to infected patients – the prevailing method then was to collect nose or throat swabs – and provided a high level of accuracy and sensitivity that is still the gold standard for coronavirus testing,” said Jay A. Tischfield, founder and CEO of RUCDR Infinite Biologics (now Infinity BiologiX LLC, or IBX, a privately owned company), Duncan and Nancy MacMillan Chair in Genetics and Distinguished Professor at Rutgers University–New Brunswick’s School of Arts and Sciences, and executive director of the Human Genetics Institute of New Jersey.
A year ago, the U.S. Food and Drug Administration gave emergency use authorization to RUCDR Infinite Biologics for the world’s first saliva-based test for the virus that has killed more than 550,000 people in the United States and millions worldwide.
“Early in the pandemic, the leadership of RUCDR recognized that accurate and easy to administer tests were needed immediately and, in collaboration with Rutgers leadership, rapidly expanded to increase testing capacity,’’ Tischfield said. “The bottom line is that Rutgers has provided a tremendous public health benefit.”

The saliva collection method developed by RUCDR, which was within the Human Genetics Institute of New Jersey, allowed for a broader population to be tested for the coronavirus than with uncomfortable nose and throat swabs.
In May 2020, the FDA granted emergency use authorization for a home collection version of the Rutgers saliva test. The lead creator of the saliva test, Andrew Brooks, who served as chief operating officer and director of technology development at RUCDR and then headed IBX, died unexpectedly in January. Brooks, 51, was also a research professor in the School of Arts and Sciences Department of Genetics at Rutgers–New Brunswick.
During a recent Rutgers podcast, Judith M. Persichilli, a Rutgers alumna and commissioner of the New Jersey Department of Health, said that at the height of the pandemic, when turnaround times for test results were five to seven days, “Rutgers ensured for the state of New Jersey that 90 percent to 95 percent of the results would be turned around in 48 to 72 hours.
That was a game changer for us,” she said. “It put us ahead of many other states in our efforts to mitigate COVID-19. So, Rutgers has been front and center in our response to this pandemic.”
Last spring, New Jersey Gov. Phil Murphy described the Rutgers saliva test as “just absolutely extraordinary work” and said an FDA-approved test that can provide rapid results was critical.
“This is a tremendous point of Jersey pride for us all,” he said. “Our state, after all, is the historic home of innovations, especially in the life sciences. Now, we have a huge breakthrough coming from our very own flagship university.”


